Abstract
Purpose: Precision oncology requires biomarker testing from tumor tissue for clinical decision-making and selection of targeted therapies. We systematically evaluated the role of tissue biomarker testing within interventional clinical trials for locally advanced and metastatic urothelial carcinoma (UC). Methods: A systematic search within the publicly available ClinicalTrials.gov database was performed for the period 1995 to January 2020. We searched for all interventional studies on systemic treatments for advanced UC. Two investigators independently screened the records and extracted the data for statistical analyses. Results: We included 356 studies out of 827 initial records in the final analysis. The overall number of interventional trials in UC patients significantly increased during the past 25 years. Forty-three studies (12.1%) required specific biomarker testing as a prerequisite for inclusion. Of the remaining 313 trials, explorative biomarkers of interest were studied in 83 studies (23.3%). In trials with obligate biomarker testing as a precondition for study inclusion, only 3 studies (7%) required an actual fresh pretreatment biopsy, while the majority of studies did not state any tissue requirements (55.8%) or accepted archival tissue samples (37.3%). Among studies without biomarker prerequisites, freshly obtained tissue samples were required in 16.3% of studies evaluating immune checkpoint inhibition and 5.7% evaluating targeted therapy. The collection of archival tissue was allowed in 67.4% and 20% of studies evaluating immune checkpoint inhibitors and targeted therapies, respectively. Conclusion: There has been an increase in the number of studies using biomarker-guided interventions for the treatment of advanced UC over the past 25 years. Studies investigating druggable targets in actual UC biopsies immediately before treatment are still rare. Standardized criteria for tissue-based biomarker testing may further accelerate personalized treatment of patients with advanced UC.
Introduction
Urothelial carcinoma (UC) of the bladder and upper urinary tract is a heterogeneous disease with high global incidence rates [1]. The prognosis of advanced UC is poor, with high rates of recurrence and progression and limited treatment options, resulting in high morbidity and mortality. Molecular research on UC has improved the understanding of tumor progression and metastasis [2]. Systemic targeted treatment alone or as part of multimodal strategies is increasingly used for patients with advanced UC [3]. Comprehensive molecular profiling with cellular and functional analyses of the tumor is important to determine specific targeted abnormalities or biomarkers that lead to better risk stratification and identification of appropriate treatments for individual patients. Large-scale genomic and transcriptomic studies were able to identify targetable alterations and are currently being translated into clinical practice to improve personalized healthcare. For example, mutational and gene expression profiling led to the discovery of genetically different molecular tumor subtypes correlating with different levels of cancer aggressiveness and response to specific treatments [4‒11].
Advanced and metastatic UC represents a heterogeneous disease, and molecular features may significantly vary between different patients and study cohorts, with dynamic changes in the course of treatment due to selection pressure [12‒15]. Thus, results of different clinical trials are hardly comparable if actual molecular characterization and stratification are lacking [7]. Although it is well known that UC can undergo significant changes during metastatic evolution [14‒16], obtaining fresh tissue material for biomarker testing immediately before treatment has rarely been performed in routine practice. However, to achieve an optimal predictive performance of tissue-based biomarkers, it may be important to consider these current molecular characteristics in freshly obtained metastatic tissue samples, as it is done in other cancer entities, for example, in breast cancer.
The advent of immunotherapies and novel targeted therapies in the advanced UC setting has been accompanied by novel and promising, mainly tissue-based biomarkers. Given the exciting evolution of current precision oncology, we conducted the present systematic analysis of interventional clinical trials to describe past developments and the current state of tissue biomarker guidance in the treatment of advanced UC.
Material and Methods
This analysis focused on the extent of biomarker testing in clinical trials studying advanced UC that are registered in ClinicalTrials.gov. The ClinicalTrials.gov registry comprises research studies worldwide and currently contains datasets on 379,473 research studies in 50 US states and in 220 countries. It is provided by the US National Library of Medicine (http://www.clinicaltrials.gov).
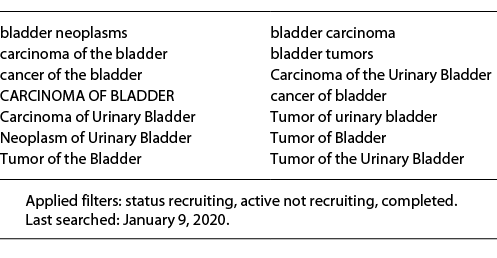
We systematically searched this publicly available database for interventional studies on systemic tumor therapy for non-operable and/or locally advanced (>T2) and/or metastatic (M1; according to the 8th version of the TNM classification system of the UICC) UC of the bladder or upper urinary tract conducted between 1995 and January 2020 (including all studies started in 2019). We used the search function provided by ClinicalTrials.gov, which expands the search strategy according to an internal algorithm (last searched: January 9, 2020). Table 1 summarizes details on the comprehensive search strategy.
Two independent investigators (F.K., M.E.) screened the records and included only active or completed studies. We excluded non-interventional or observational studies, studies not including UC, or studies evaluating nonsystemic or intravesical therapies. Basket trials were included if inclusion of UC patients was possible.
The two investigators independently extracted data, and disagreement was resolved by discussion or by consultation with a third investigator (CB) if required. To facilitate interpretation of screened and included studies, we categorized study designs and interventions as follows: (1) studies requiring an obligate biomarker testing (e.g., Her2neu testing) versus studies not requiring baseline biomarker testing as prerequisite for study participation (exploratory biomarker testing). (2) Mode of treatment: targeted therapy with(out) chemotherapy; immune checkpoint inhibition with(out) other treatment modality; adaptive cell therapy or cancer vaccines or oncolytic viruses or immune modulation (excluding immune checkpoint inhibition); targeted chemotherapy or antibody-drug conjugates; conventional chemotherapy; gene therapy. (3) Requirements for tissue collection and biomarker testing: requirement of fresh pretreatment/baseline tissue; requirement of archival or freshly acquired tissue (if no archival tissue is available); requirement of archival tissue only; no tissue requirements stated per study protocol. Where appropriate, methodology was designed, conducted, and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [17]. We descriptively analyzed the results. Statistical analysis was performed with JMP SAS 13.2 (SAS Institute, Cary, NC, USA). An ethics statement is not applicable because this study is based exclusively on published literature.
Results
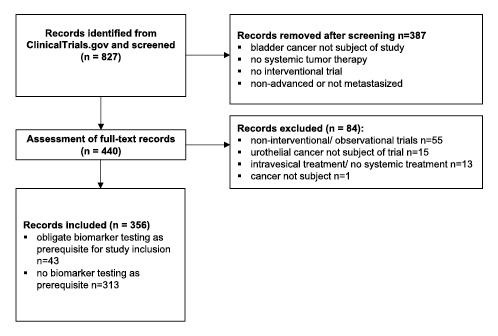
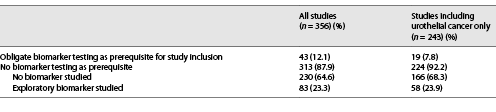
A total of 827 studies were identified through database searching. After title screening, we assessed full-texts of 440 studies for eligibility and finally included 356 studies. Figure 1 illustrates the search workflow. Of 356 included records, 43 (12.1%) studies required an obligate biomarker testing as prerequisite for study inclusion and 313 (87.9%) did not (Fig. 1).
Study Characteristics
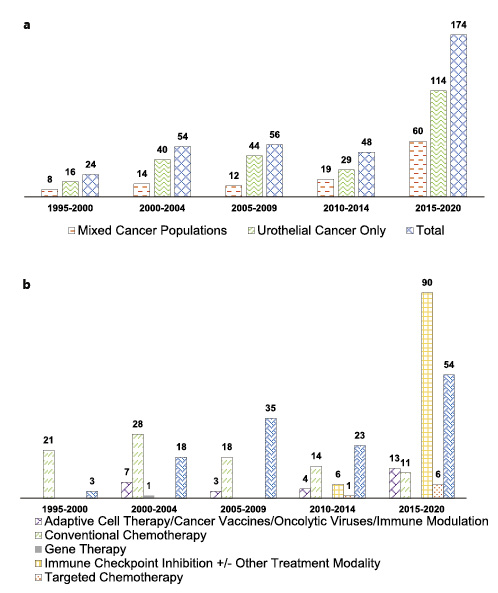
Of the 356 included studies, 113 (31.7%) enrolled mixed cancer populations, and 243 (68.3%) enrolled only participants with UC. The total number of studies allowing enrollment of UC has steadily increased over the past 25 years (Fig. 2a). Of all 356 studies, 27 (7.6%) studies evaluated adaptive cell therapy/cancer vaccines/oncolytic viruses or immune modulation, 1 (0.3%) study evaluated gene therapy, 7 (2%) studies evaluated targeted chemotherapy/antibody-drug conjugates, 92 (25.8%) studies evaluated conventional chemotherapy, 96 (27%) studies evaluated immune checkpoint inhibition with(out) other treatment modalities, and 133 (37.4%) studies evaluated targeted therapy with(out) chemotherapy (Table 2). We identified no differences in the rate of biomarker testing between studies including UC of the bladder only (Table 3).
a Total number of studies recruiting patients with urothelial cancer since 1995. b Included studies stratified according to mode of treatment.
a Total number of studies recruiting patients with urothelial cancer since 1995. b Included studies stratified according to mode of treatment.
While the number of conventional chemotherapy trials decreased between 1995 and 2020, trials evaluating immune checkpoint inhibition or targeted therapy increased steadily (Fig. 2b). Trials exploring targeted therapies or adaptive cell therapies, cancer vaccines, oncolytic viruses, or other immune modulators remained at stable levels between 1995 and 2020 (Fig. 2b).
Biomarker Testing/Exploration and Tissue Requirements for Biomarker Testing/Exploration
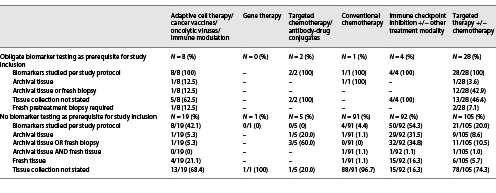
Besides proper methodological biomarker testing, the choice of the “right” tissue specimen for testing is crucial for obtaining valid results due to well-known processes of genetic and transcriptomic/proteomic tumor evolution during local progression or metastatic evolution of many cancer entities. Thus, we further analyzed which tissue sample requirements were stated in study descriptions on ClinicalTrials.gov. Remarkably, of 43 studies requiring specific biomarker constellations for study inclusion, only 3 (7.0%) required a fresh pretreatment biopsy for testing of biomarkers with known volatility (online suppl. Table 1; for online suppl material, see www.karger.com/doi/10.1159/000527879). Usage of either archival tissue or freshly acquired tissue biopsies (if no archival tissue was available) was allowed in 14 studies (32.6%). Two studies (4.7%) required archival tissue, and in 24 (55.8%) studies, no specific requirements for tissue collection were stated (Table 4).
Summary of tissue requirements as stated per study protocol on “ClinicalTrials.gov” in analyzed clinical trials
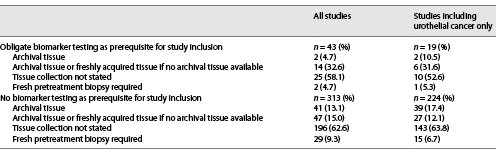
Of 356 studies, 43 (12.1%) required an obligate biomarker testing as a prerequisite for study inclusion (Table 3). The most frequently tested biomarkers were overexpression of Her2neu on protein level and ERBB2 gene amplifications. Online supplementary Table 1 contains a comprehensive summary of these 43 studies, including details on required biomarkers. Biomarker testing was not required as a prerequisite for study inclusion in 313 studies (87.9%; Table 3). Remarkably, exploratory biomarkers of interest were assessed in only 83 (26.5%) of these 313 studies. However, as biomarkers may be of secondary importance (e.g., for conventional chemotherapy tolerability/comparative trials) or of utmost importance (targeted therapies, immunotherapies) in different therapy settings, we further analyzed to what extent exploratory biomarkers were investigated within specific study settings. While testing/exploration rates for conventional chemotherapy trials were predictably low at 4.4%, rates of 54.3% for immune checkpoint inhibitor trials and 20.0% for targeted therapy trials were surprisingly low (Table 5).
In the 313 studies without biomarker testing as a prerequisite for study inclusion, tissue samples were collected per study protocol in 37.3% of all studies (archival tissue: 13.1%, archival tissue or freshly acquired tissue if no archival tissue was available: 15%, fresh pretreatment biopsy required: 9.3%; Table 4, 5). Interestingly, for specific study settings, the rate of tissue collection was higher than the rate of biomarker exploration/assessment, as stated on ClinicalTrials.gov (e.g., immune checkpoint inhibitor trials: biomarkers studied in 54.3% vs. tissue collected in 83.7% of studies; Table 5).
Furthermore, it is important to note that acquisition of fresh pretreatment biopsies (4.7% of studies with biomarker prerequisites, 9.3% of studies without biomarker prerequisites) was remarkably low (Table 4, 5). Especially for trials with biomarker prerequisites, the rate of 4.7% for obtaining fresh pretreatment biopsies for assessment of requested biomarkers was extremely low. However, for studies without biomarker prerequisites, overall rates are not representative of specific study settings where biomarker testing is more important than in other settings.
As summarized in Table 5, however, collection rates of fresh pretreatment/baseline biopsies were also extremely low in immune checkpoint inhibitor trials (16.3%) and trials studying targeted therapies (5.7%), where the predictive potential of specific biomarkers has been explored in the past (e.g., expression of PD-L1 on immune and/or tumor cells).
Discussion
Biomarker testing is necessary for individualized systemic treatment. Here, we evaluated the role of biomarker testing in interventional studies including participants with advanced UC that were registered in ClinicalTrials.gov. The total number of studies including patients with UC has continuously increased during the past 25 years. The highest increase in terms of total available trials was observed after 2015, when most of the large immunotherapy trials in advanced UC were initiated. UC is still a relatively neglected urological malignancy when compared to other cancers like breast or lung cancer and other urological cancer types like prostate and kidney cancer. However, several years ago, a steady increase began in the numbers of new publications per year for UC several years ago [18]. Currently, a decline of conventional chemotherapy trials in advanced UC can be observed, while studies investigating novel targeted or immunotherapeutic agents accounted for the majority of newly registered trials.
In the new era of precision oncology, an evaluation of specific druggable targets is becoming more and more critical. However, the majority of studies including immunotherapy or targeted therapy trials did not state in the study protocol on “ClinicalTrials.gov” whether biomarker testing or exploration was needed. Importantly, in studies with an obligate biomarker testing as a prerequisite for study inclusion, only 7.0% required a fresh pretreatment biopsy, and the majority of studies did not provide any information on how tissue collection should be carried out. In studies with no required biomarker testing as prerequisite for study inclusion, biomarkers were explored in a substantial number of trials, especially in studies exploring the efficacy of novel immunotherapies (mainly immune checkpoint inhibitors). However, the majority of immune checkpoint inhibitor trials allowed the collection of archival tissue, and only a minority required fresh pretreatment tissue biopsies. This has to be seen very critically in the background of past studies, which showed that immunological biomarkers like PD-L1 are very volatile and can differ greatly between primary and metastatic tumor sites [16]. Although this has not been proven systematically yet, this “primary-to-distant heterogeneity and volatility” might be an important contributor to the PD-L1 biomarker “dilemma” in advanced UC [19].
One especially interesting example among the immunotherapy trials is the MAGNOLIA trial. Based on the observation that MAGE-A3 antigen expression in UC is associated with poor prognosis, the MAGNOLIA trial recruited histologically confirmed MAGE-A3 positive UC of the bladder and aimed to evaluate the safety and efficacy of adjuvant recombinant MAGE-A3 immunotherapy in combination with an immunostimulant after cystectomy for muscle-invasive bladder cancer. MAGE-A3 positivity was a prerequirement for study inclusion, and biological samples (tissue, blood, serum, and urine) were therefore collected for translational research [20]. However, the trial recruitment was stopped early because of negative outcomes in trials evaluating melanoma and lung cancer [20]. Consequently, the MAGNOLIA trial was not able to investigate the potential efficacy of anti-MAGE-A3 therapy in UC.
Translational research is the backbone of clinical scientific investigations, and it is a positive signal that biomarker testing in UC studies has been increasing during the last 25 years. However, the majority of studies did not state a biomarker testing in their study protocols, and even half of studies evaluating novel therapy concepts such as targeted therapy and immune checkpoint inhibition did not present information on biomarker testing on ClinicalTrials.gov. It is well known that the molecular characteristics of bladder cancer change during the time course of advanced disease (e.g., volatile immunological biomarkers such as PD-L1) [16], and that it is actually necessary to take a fresh tissue sample from the tumor – or even better – from a metastatic lesion [5, 21‒25]. In other cancer types (e.g., breast cancer), it is good clinical practice to rebiopsy recurring or progressing disease because it is well known that the molecular state of the tumor can change and targetable molecular alteration can be found in recent biopsies due to selection by previous therapy. However, only 7% of studies with required biomarker testing demanded a fresh pretreatment biopsy that reflects the current state of disease. Pretreatment biopsies in advanced or metastatic UC must be explicitly addressed and supported.
We found that 12% of all studies evaluating systemic tumor therapy in patients with advanced or metastatic UC required an obligate biomarker testing as a prerequisite for study inclusion. There is no information in biomedical databases on the number of studies with required biomarker testing in other cancer types. It, therefore, remains unclear if this percentage is comparable in bladder cancer. However, from a translational point of view, it would be worthwhile to increase this number. On the other hand, in studies with no required biomarker testing as a prerequisite for study inclusion, 23.3% of studies tested, nevertheless, a biomarker; also, 37.3% of studies collected tissue (fresh and/or archival tissue). There is, theoretically, more tissue available for translational research.
The present report has potential limitations. Studies evaluating advanced or metastatic UC were assessed in the ClinicalTrials.gov database. It is possible that further trials requiring biomarker testing are currently underway, but they have not been registered in this database. Another limitation includes that all trials were analyzed independent of their completion status (also potentially failed studies or studies which never generated relevant results). We, however, assume that our evaluation is representative of the actual study landscape in UC research (time span 1995–2020) and that the amount of biomarker testing in non-muscle-invasive bladder cancer might be even smaller. However, UC is a highly heterogeneous disease, and it is well known that current classification systems do not cover the entire disease [12]. Current research has improved our understanding of UC biology, leading to the identification of several molecular subtypes that might predict treatment response [12, 26]. Interaction of DNA damage and DNA repair mechanisms may predict treatment response and provide the possibility of further therapy approaches [10, 27‒29]. Changes in DNA repair signaling pathways using FGFR or PARP inhibitors are currently being tested in clinical trials [30]. The biological meaning of these molecular subtypes has not thoroughly been considered and – in order to move toward precision medicine – it might be worthwhile to ensure these findings by further biomarker testing in clinical trials.
Conclusion
In conclusion, we present a comprehensive overview on the clinical trial landscape of biomarker-based clinical trials for patients with advanced UC. More and more studies using biomarker-guided interventions for the treatment of advanced UC were recently initiated. However, studies investigating druggable targets in actual UC biopsies are still rare and need to be promoted.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
All authors declare no direct conflicts of interest related to this work. Potential unrelated conflicts of interest: Christian Bolenz – speaker’s honoraria from Janssen-Cilag, AstraZeneca, medac, Takeda, Roche, and Ipsen; advisory role for Roche and ERBE; Arndt Hartmann – honoraria for lectures for Abbvie, AstraZeneca, Biontech, BMS, Boehringer Ingelheim, Cepheid, Diaceutics, Ipsen, Janssen, Lilly, MSD, Nanostring, Novartis, Roche, and 3DHistotech; advisory role for Abbvie, AstraZeneca, Biontech, BMS, Boehringer Ingelheim, Cepheid, Diaceutics, Gilead, Illumina, Ipsen, Janssen, Lilly, MSD, Nanostring, Novartis, Qiagen, QUIP GmbH, Roche, and 3DHistotech; research funding from AstraZeneca, Biontech, Cepheid, Gilead, Illumina, Janssen, Nanostring, Qiagen, QUIP GmbH, Roche, and STRATIFYER; Markus Eckstein – personal fees, travel costs, and speaker’s honoraria from MSD, AstraZeneca, Janssen-Cilag, Cepheid, Roche, Astellas, and Diaceutics; research funding from AstraZeneca, Janssen-Cilag, STRATIFYER, Cepheid, Roche, and Gilead; advisory roles for Diaceutics, MSD, AstraZeneca, Janssen-Cilag, and GenomicHealth.
Funding Sources
The project was funded and supported by the general scientific funding pool of each participating department. Markus Eckstein was supported by the IZKF of the FAU Erlangen-Nürnberg (clinician scientist program to Markus Eckstein) and the Else Kröner-Fresenius-Stiftung (Grant number: 2020_EKEA.129).
Author Contributions
Study conceptualization: all contributing authors. Study management: Frank Kunath, Friedemann Zengerling, Felix Wezel, Stephanie Schmidt, and Markus Eckstein. Independent supervision and data monitoring (direct access and verification of the underlying data reported in the manuscript): Christian Bolenz and Arndt Hartmann. Administrative, technical, or material/data support: Stephanie Schmidt. Assembly of the cohort and clinical data extraction: Stephanie Schmidt, Frank Kunath, and Markus Eckstein. Statistical analysis, bioinformatics, and supervision: Markus Eckstein and Frank Kunath. Manuscript writing: Markus Eckstein, Frank Kunath, and Christian Bolenz. Critical revision of the manuscript for important intellectual content: all contributing authors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
References
Additional information
Christian Bolenz and Frank Kunath contributed equally to this work.